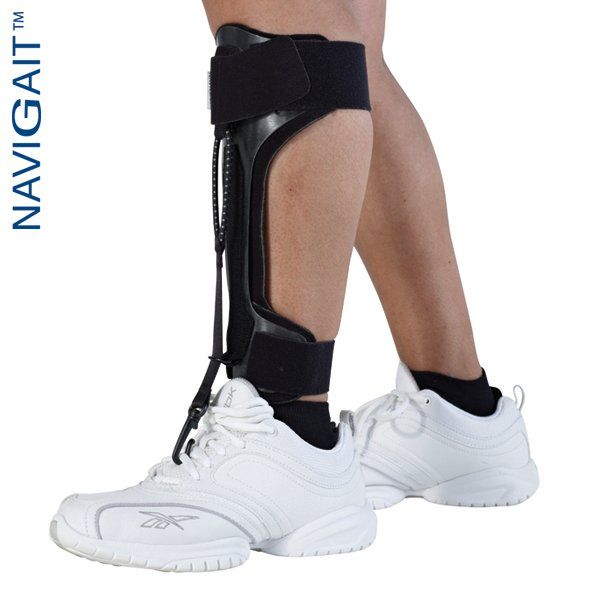
TOEOFF® Flow 2½
TOEOFF® Flow 2½
For your active patients or others who require less resistance or more ROM. We applied Allard’s 20+ years of AFO carbon composite experience and advances in technology to create a new proprietary formula to meet the functional and comfort needs of even more individuals.
The new FLOW technology offers a smoother transition (flow) throughout the gait cycle and has increased ROM in the sagittal plane. ToeOFF® FLOW 2½ has a lower heel height (approx. 7mm) which accommodates gentler contours of shoe insoles and offers more clearance for the forefoot in the shoe toe box. It is easier to customize to meet alignment and biomechanical needs of your patients.
Recommended Range of Application:
Foot drop combined with 0 to moderate spasticity, in some cases even with impaired knee control and toe amputation. Limb proprioception deficit and mild proximal deficit. For further information on uses and contraindications see AFO Professional Instructions.
Contraindications:
ToeOFF® FLOW 2½ should not be used when patients present with foot and/or leg ulcers, moderate to severe edema, moderate to severe foot deformities, severe proximal deficits (e.g. quadriceps spasticity, genu valgum or varum, genu recurvatum), or severe spasticity.
Other:
ToeOFF® FLOW 2½ includes pre-applied MikroFIX™, upper and lower straps, and a Starter Interface (one SoftKIT™ pad and two vertical tibia pads). Optional padding kits include; ComfortKIT™, GliderKIT™, and CoverKIT™ interfaces which are sold separately. More information on testing, adaptation, choice of model and size can be found in Allard AFO Professional Instructions.